Characteristic infrared intensities of carbonyl stretching vibrations†
Abstract
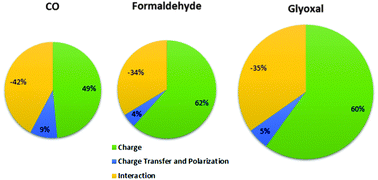
The experimental infrared fundamental intensities of gas phase carbonyl compounds obtained by the integration of spectral bands in the Pacific Northwest National Laboratory (PNNL) spectral database are in good agreement with the intensities reported by other laboratories having a root mean square error of 27 km mol−1 or about 13% of the average intensity value. The Quantum Theory of Atoms in Molecules/Charge–Charge Transfer–Counterpolarization (QTAIM/CCTCP) model indicates that the large intensity variation from 61.7 to 415.4 km mol−1 is largely due to static atomic charge contributions, whereas charge transfer and counterpolarization effects essentially cancel one another leaving only a small net effect. The Characteristic Substituent Shift Model estimates the atomic charge contributions to the carbonyl stretching intensities within 30 km mol−1 or 10% of the average contribution. However, owing to the size of the 2 × C × CTCP interaction contribution, the total intensities cannot be estimated with this degree of accuracy. The dynamic intensity contributions of the carbon and oxygen atoms account for almost all of the total stretching intensities. These contributions vary over large ranges with the dynamic contributions of carbon being about twice the size of the oxygen ones for a large majority of carbonyls. Although the carbon monoxide molecule has an almost null dipole moment contrary to the very polar bond of the characteristic carbonyl group, its QTAIM/CCTCP model is very similar to those found for the carbonyl compounds.

 Please wait while we load your content...
Please wait while we load your content...