A through-space description of substituent effects leads to inaccurate molecular electrostatic potentials and cation⋯π interactions in extended aromatic systems†
Abstract
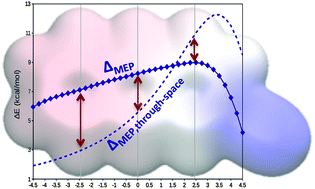
Non-local effects are crucial in order to give an accurate description of substituent effects in extended aromatic systems. As a consequence, the predictions based on the currently accepted through-space picture can lead to large errors in the strength of cation⋯π interactions, especially for rings furthest from the substituent.

 Please wait while we load your content...
Please wait while we load your content...