Structural effect of glyme–Li+ salt solvate ionic liquids on the conformation of poly(ethylene oxide)†
Abstract
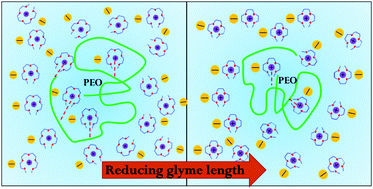
The conformation of 36 kDa polyethylene oxide (PEO) dissolved in three glyme–Li+ solvate ionic liquids (SILs) has been investigated by small angle neutron scattering (SANS) and rheology as a function of concentration and compared to a previously studied SIL. The solvent quality of a SIL for PEO can be tuned by changing the glyme length and anion type. Thermogravimetric analysis (TGA) reveals that PEO is dissolved in the SILs through Li+–PEO coordinate bonds. All SILs (lithium triglyme bis(trifluoromethanesulfonyl)imide ([Li(G3)]TFSI), lithium tetraglyme bis(pentafluoroethanesulfonyl)imide ([Li(G4)]BETI), lithium tetraglyme perchlorate ([Li(G4)]ClO4) and the recently published [Li(G4)]TFSI) are found to be moderately good solvents for PEO but solvent quality decreases in the order [Li(G4)]TFSI ∼ [Li(G4)]BETI > [Li(G4)]ClO4 > [Li(G3)]TFSI due to decreased availability of Li+ for PEO coordination. For the same glyme length, the solvent qualities of SILs with TFSI− and BETI− anions ([Li(G4)]TFSI and [Li(G4)]BETI) are very similar because they weakly coordinate with Li+, which facilitates Li+–PEO interactions. [Li(G4)]ClO4 presents a poorer solvent environment for PEO than [Li(G4)]BETI because ClO4− binds more strongly to Li+ and thereby hinders interactions with PEO. [Li(G3)]TFSI is the poorest PEO solvent of these SILs because G3 binds more strongly to Li+ than G4. Rheological and radius of gyration (Rg) data as a function of PEO concentration show that the PEO overlap concentrations, c* and c**, are similar in the three SILs.

 Please wait while we load your content...
Please wait while we load your content...