Fullerenol C60(OH)16 prevents amyloid fibrillization of Aβ40 – in vitro and in silico approach†
Abstract
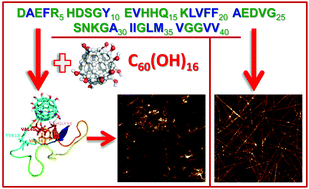
The generation of Aβ amyloid aggregates in the form of senile plaques in the brain is one of the pathological hallmarks of Alzheimer's disease (AD). There is no cure for AD and one of the recent treatment strategies is focused on the inhibition of amyloid fibrillization of Aβ peptide. Fullerene C60 has been proposed as a candidate for destroying Aβ aggregates but it is not soluble in water and its toxicity to cells remains largely ambiguous. To overcome these drawbacks, we synthesized and studied the effect of water-soluble fullerenol C60(OH)16 (fullerene C60 carrying 16 hydroxyl groups) on the amyloid fibrillization of Aβ40 peptide in vitro. Using a Thioflavin T fluorescent assay and atomic force microscopy it was found that C60(OH)16 effectively reduces the formation of amyloid fibrils. The IC50 value is in the low range (μg ml−1) suggesting that fullerenol interferes with Aβ40 aggregation at stoichiometric concentrations. The in silico calculations supported the experimental data. It was revealed that fullerenol tightly binds to monomer Aβ40 and polar, negatively charged amino acids play a key role. Electrostatic interactions dominantly contribute to the binding propensity via interaction of the oxygen atoms from the COO− groups of side chains of polar, negatively charged amino acids with the OH groups of fullerenol. This stabilizes contact with either the D23 or K28 of the salt bridge. Due to the lack of a well-defined binding pocket fullerenol is also inclined to locate near the central hydrophobic region of Aβ40 and can bind to the hydrophobic C-terminal of the peptide. Upon fullerenol binding the salt bridge becomes flexible, inhibiting Aβ aggregation. In order to assess the toxicity of fullerenol, we found that exposure of neuroblastoma SH-SY5Y cells to fullerenol caused no significant changes in viability after 24 h of treatment. These results suggest that fullerenol C60(OH)16 represents a promising candidate as a therapeutic for Alzheimer's disease.

 Please wait while we load your content...
Please wait while we load your content...