Identification and H(D)-bond energies of C–H(D)⋯Cl interactions in chloride–haloalkane clusters: a combined X-ray crystallographic, spectroscopic, and theoretical study†
Abstract
The cationic (1,3,5-triazapentadiene)PtII complex [Pt{NH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(N(CH2)5)N(Ph)C(NH2)
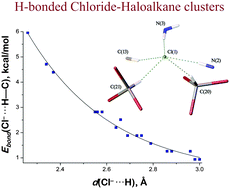
C(N(CH2)5)N(Ph)C(NH2)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NPh}2]Cl2 ([1]Cl2) was crystallized from four haloalkane solvents giving [1][Cl2(CDCl3)4], [1][Cl2(CHBr3)4], [1][Cl2(CH2Cl2)2], and [1][Cl2(C2H4Cl2)2] solvates that were studied by X-ray diffraction. In the crystal structures of [1][Cl2(CDCl3)4] and [1][Cl2(CHBr3)4], the Cl− ion interacts with two haloform molecules via C–D⋯Cl− and C–H⋯Cl− contacts, thus forming the negatively charged isostructural clusters [Cl(CDCl3)2]− and [Cl(CHBr3)2]−. In the structures of [1][Cl2(CH2Cl2)2] and [1][Cl2(C2H4Cl2)2], cations [1]2+ are linked to a 3D-network by a system of H-bondings including one formed by each Cl− ion with CH2Cl2 or C2H4Cl2 molecules. The lengths and energies of these H-bonds in the chloride–haloalkane clusters were analyzed by DFT calculations (M06 functional) including AIM analysis. The crystal packing noticeably affected the geometry of the clusters, and energy of C–H⋯Cl− hydrogen bonds ranged from 1 to 6 kcal mol−1. An exponential correlation (R2 > 0.98) between the calculated Cl−⋯H distances and the energies of the corresponding contacts was found and used to calculate hydrogen bond energies from the experimental Cl−⋯H distances. Predicted energy values (3.3–3.9 kcal mol−1 for the [Cl(CHCl3)2]− cluster) are in a reasonable agreement with the energy of the Cl3C–H⋯Cl− bond estimated using ATRFTIR spectroscopy (2.7 kcal mol−1).
NPh}2]Cl2 ([1]Cl2) was crystallized from four haloalkane solvents giving [1][Cl2(CDCl3)4], [1][Cl2(CHBr3)4], [1][Cl2(CH2Cl2)2], and [1][Cl2(C2H4Cl2)2] solvates that were studied by X-ray diffraction. In the crystal structures of [1][Cl2(CDCl3)4] and [1][Cl2(CHBr3)4], the Cl− ion interacts with two haloform molecules via C–D⋯Cl− and C–H⋯Cl− contacts, thus forming the negatively charged isostructural clusters [Cl(CDCl3)2]− and [Cl(CHBr3)2]−. In the structures of [1][Cl2(CH2Cl2)2] and [1][Cl2(C2H4Cl2)2], cations [1]2+ are linked to a 3D-network by a system of H-bondings including one formed by each Cl− ion with CH2Cl2 or C2H4Cl2 molecules. The lengths and energies of these H-bonds in the chloride–haloalkane clusters were analyzed by DFT calculations (M06 functional) including AIM analysis. The crystal packing noticeably affected the geometry of the clusters, and energy of C–H⋯Cl− hydrogen bonds ranged from 1 to 6 kcal mol−1. An exponential correlation (R2 > 0.98) between the calculated Cl−⋯H distances and the energies of the corresponding contacts was found and used to calculate hydrogen bond energies from the experimental Cl−⋯H distances. Predicted energy values (3.3–3.9 kcal mol−1 for the [Cl(CHCl3)2]− cluster) are in a reasonable agreement with the energy of the Cl3C–H⋯Cl− bond estimated using ATRFTIR spectroscopy (2.7 kcal mol−1).
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...