Hückel–Hubbard–Ohno modeling of π-bonds in ethene and ethyne with application to trans-polyacetylene
Abstract
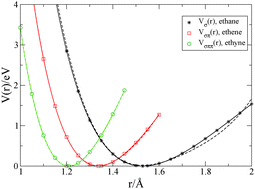
Quantum chemistry calculations provide the potential energy between two carbon atoms in ethane (H3C–CH3), ethene (H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), and ethyne (HC
CH2), and ethyne (HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH) as a function of the atomic distance. Based on the energy function for the σ-bond in ethane, Vσ(r), we use the Hückel model with Hubbard–Ohno interaction for the π electrons to describe the energies Vσπ(r) and Vσππ(r) for the σπ double bond in ethene and the σππ triple bond in ethyne, respectively. The fit of the force functions shows that the electron transfer matrix element and the Peierls coupling can be estimated with some precision whereas the Hubbard–Ohno parameters are insignificant at the distances under consideration. We apply the Hückel–Hubbard–Ohno model to describe the bond lengths and the energies of elementary electronic excitations of trans-polyacetylene, (CH)n, whereby we adjust the σ-bond potential for conjugated polymers.
CH) as a function of the atomic distance. Based on the energy function for the σ-bond in ethane, Vσ(r), we use the Hückel model with Hubbard–Ohno interaction for the π electrons to describe the energies Vσπ(r) and Vσππ(r) for the σπ double bond in ethene and the σππ triple bond in ethyne, respectively. The fit of the force functions shows that the electron transfer matrix element and the Peierls coupling can be estimated with some precision whereas the Hubbard–Ohno parameters are insignificant at the distances under consideration. We apply the Hückel–Hubbard–Ohno model to describe the bond lengths and the energies of elementary electronic excitations of trans-polyacetylene, (CH)n, whereby we adjust the σ-bond potential for conjugated polymers.

 Please wait while we load your content...
Please wait while we load your content...