Phase behaviour and conductivity of supporting electrolytes in supercritical difluoromethane and 1,1-difluoroethane†
Abstract
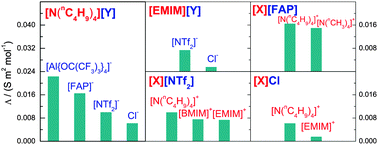
We present investigations into a variety of supporting electrolytes and supercritical fluids probing the phase and conductivity behaviour of these systems and show that they not only provide sufficient electrical conductivity for an electrodeposition bath, but match the requirements imposed by the different precursors and process parameters, e.g. increased temperature, for potential deposition experiments. The two supercritical fluids that have been explored in this study are difluoromethane (CH2F2) and 1,1-difluoroethane (CHF2CH3). For CH2F2, the phase behaviour and electrical conductivity of eight ionic compounds have been studied. Each compound consists of a cation and an anion from the selected candidates i.e. tetramethylammonium ([N(CH3)4]+), tetrabutylammonium ([N(nC4H9)4]+), 1-ethyl-3-methylimidazolium ([EMIM]+) and 1-butyl-3-methylimidazolium ([BMIM]+) for cations, and tetrakis(perfluoro-tert-butoxy)aluminate ([Al(OC(CF3)3)4]−), chloride (Cl−), trifluoromethyl sulfonimide ([NTf2]−) and tris(pentafluoroethyl)trifluorophosphate ([FAP]−) for anions. For CHF2CH3, [N(nC4H9)4][BF4] and [N(nC4H9)4][B{3,5-C6H3(CF3)2}4] have been investigated for comparison with the previously measured solubility and conductivity in CH2F2. We have found that [N(nC4H9)4][Al(OC(CF3)3)4], [N(nC4H9)4][FAP] and [N(CH3)4][FAP] have much higher molar conductivity in scCH2F2 at similar conditions than [N(nC4H9)4][BF4], a widely used commercial electrolyte. Additionally, scCHF2CH3 shows potential for use as the solvent for supercritical fluid electrodeposition, especially at high temperatures since high density of this fluid can be achieved at lower operating pressures than similar fluids that can be used to produce electrochemical baths with comparable conductivity.

 Please wait while we load your content...
Please wait while we load your content...