The self-assembly of copolymers with one hydrophobic and one polyelectrolyte block in aqueous media: a dissipative particle dynamics study†
Abstract
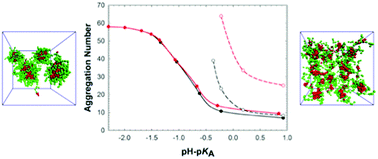
The reversible self-assembly of symmetrical block copolymers consisting of one hydrophobic block and one ionizable polyelectrolyte block of the same length has been studied in aqueous solutions by dissipative particle dynamics simulations. In addition to three standard dissipative particle dynamics forces (conservative soft repulsion, dissipative and stochastic forces), explicit interaction between smeared charges on ions and on ionized polymer beads described by the electrostatic potential with appropriately localized charges was taken into account. The self-assembly and properties of formed core–shell micelles were investigated as functions of the degree of ionization for systems differing in the hydrophobicity of the non-ionized polyelectrolyte block and in the compatibility of the polymer blocks. This study shows that micelles undergo massive dissociation with increasing degree of ionization. The simulation data compare well with the predictions of scaling theories for systems with soluble polyelectrolytes on a semi-quantitative level and broaden the knowledge of systems in poor solvents.

 Please wait while we load your content...
Please wait while we load your content...