Effects of the position and number of bromine substituents on the concentration-mediated 2D self-assembly of phenanthrene derivatives†
Abstract
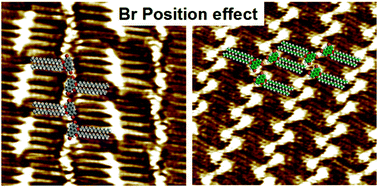
The effects of the position and number of bromine substituents on the self-assembled patterns of phenanthrene derivatives by changing multiple weak intermolecular interactions were investigated at the 1-octanoic acid/graphite interface at different concentrations by scanning tunneling microscopy. Two Br substituted DBHP molecules (2,7-DBHP, 3,6-DBHP) and BHP without a Br group formed a linear lamellar pattern by the van der Waals interactions between the alkoxyl chains in each lamella at high concentrations, which forces the phenanthrene derivatives to self-organize in a π–π stacked edge-on conformation. On decreasing the solution concentration, owing to the molecule–molecule van der Waals force and Br⋯Br halogen bonds or the molecule–solvent cooperative Br⋯O (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) hydrogen and Br⋯HO–hydrogen bonds, 2,7-DBHP molecules were found to form two kinds of network structures, whereas 3,6-DBHP molecules formed only a zigzag pattern due to the intermolecular Br⋯Br van der Waals type interactions. One bromine substituted phenanthrene derivative (3-DBHP) formed a dislocated linear pattern by two C–H⋯Br hydrogen bonds in each dimer. These observations revealed that an important modification of the position and number of halogen substituents might dramatically change the self-assembly behaviors by different intermolecular interactions including Br⋯Br and Br⋯O halogen bonding, Br⋯Br van der Waals type interactions, and H⋯Br hydrogen bonding. DFT calculations were explored to unravel how slightly tuning the molecular structure defines the geometry of a 2D self-assembled nanoarchitecture through the different elementary structural units having Br⋯Br and Br⋯H interactions.
O) hydrogen and Br⋯HO–hydrogen bonds, 2,7-DBHP molecules were found to form two kinds of network structures, whereas 3,6-DBHP molecules formed only a zigzag pattern due to the intermolecular Br⋯Br van der Waals type interactions. One bromine substituted phenanthrene derivative (3-DBHP) formed a dislocated linear pattern by two C–H⋯Br hydrogen bonds in each dimer. These observations revealed that an important modification of the position and number of halogen substituents might dramatically change the self-assembly behaviors by different intermolecular interactions including Br⋯Br and Br⋯O halogen bonding, Br⋯Br van der Waals type interactions, and H⋯Br hydrogen bonding. DFT calculations were explored to unravel how slightly tuning the molecular structure defines the geometry of a 2D self-assembled nanoarchitecture through the different elementary structural units having Br⋯Br and Br⋯H interactions.

 Please wait while we load your content...
Please wait while we load your content...