Quantum chemical clarification of the alkyl chain length threshold of nonionic surfactants for monolayer formation at the air/water interface
Abstract
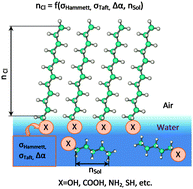
A theoretical basis is provided for the experimental fact that for various surfactant classes the alkyl chain length threshold varies for the formation of condensed monolayers. The existence of the alkyl chain length threshold for a surfactant enabling the formation of monolayers is determined by the entropy increment to the Gibbs' energy, assessed by using the quantum chemical semiempiric method PM3. The value of the clusterization threshold is not stipulated by the surfactant solubility in water, rather by the electron-donor and electron-seeking properties of the head groups. These properties in turn impact the value of the solubility threshold for surfactants. The value of the clusterization threshold depends quadratically on the substituent constants, i.e. it is independent of whether the functional group is a donor or an acceptor of electrons. Rather it depends only on the donor or the acceptor ‘force’ of the substituent. The square-law dependence of the surface clusterization threshold of the amphiphile on the solubility threshold is evidenced.

 Please wait while we load your content...
Please wait while we load your content...