First-principles studies on the structural and spectral properties of C72 isomers and the chlorinated derivative C72Cl4†
Abstract
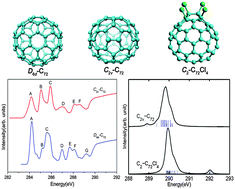
X-ray photoelectron (XPS) and near-edge X-ray absorption fine structure (NEXAFS) spectra, as well as the ground-state electronic/geometrical structures of two important isomers of the elusive C72 fullerene family (C2v- and D6d-symmetry, respectively) and the corresponding chlorinated derivative C72Cl4, which are newly captured in the experiment, have been simulated at the density functional theory (DFT) level. Effective changes in the electronic structure and simulated X-ray spectra have been observed after chlorination. Both spectra show strong isomer dependence, therefore the “fingerprints” in the X-ray spectra offer a useful method for isomer identification of the above-mentioned fullerenes. The ultraviolet-visible (UV-vis) absorption spectroscopy of C72Cl4 has also been performed by means of time-dependent (TD) DFT calculations. The simulated UV-vis spectrum is in good agreement with the experimental results. The results of this work can provide valuable information for further experimental and theoretical studies on newly synthesized fullerene isomers and their derivatives by means of X-ray and UV-vis spectroscopy techniques.

 Please wait while we load your content...
Please wait while we load your content...