Characterization of Fe2+ ions in Fe,H/SSZ-13 zeolites: FTIR spectroscopy of CO and NO probe molecules†
Abstract
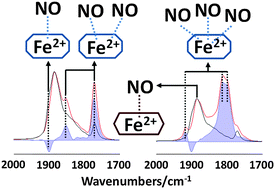
The IR spectra of adsorbed CO and NO probe molecules were used to characterize the coordination chemistry of Fe2+ ions in solution ion exchanged Fe,H/SSZ-13 zeolites. The effects of Fe ion exchange levels, as well as the sample pre-treatment conditions, on the adsorption of these probe molecules were investigated. The ion exchange levels (in the range of the study) did not affect significantly the IR spectra of either probe molecule, and the IR features and their intensity ratios were very similar. Experiments with both probe molecules substantiated the presence of two distinct types of Fe2+ ions in cationic positions. We assign these two Fe2+ ions to two distinct cationic positions: Fe2+ in 6R and 8R positions. NO initially adsorbs preferentially onto Fe2+ sites in the 6R position, and then populates sites in the 8R. Fe2+ ions in the 8R positions require the interaction of more than one NO molecule to move them out from their adsorbate-free cationic positions. As soon as they move from their stable positions, they are able to bind to multiple NO molecules, and form mostly tri-nitrosyls. These tri-nitrosyls, however, are only stable in the presence of gas phase NO; under dynamic vacuum they lose one of the NO molecules from their coordination sphere and form stable di-nitrosyls. The adsorption of CO is much weaker on Fe2+ sites than that of NO, and requires cryogenic sample temperatures to initiate CO adsorption. Under the conditions applied in this study, only mono-carbonyl formation was observed. Reduction in H2 at 773 K increased the number of Fe2+ adsorption sites, primarily in the 8R locations. Oxidation by N2O, on the other hand, selectively reduced the adsorption of both CO and NO on the Fe2+ sites in 8R positions. Adsorbed oxygen left behind from the decomposition of N2O at 573 K readily reacted with CO to produce CO2 even at 150 K.

 Please wait while we load your content...
Please wait while we load your content...