Force field development and simulations of senior dialkyl sulfoxides†
Abstract
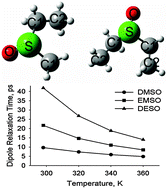
Thermodynamics, structure, and dynamics of diethyl sulfoxide (DESO) and ethyl methyl sulfoxide (EMSO) were investigated using ab initio calculations and non-polarizable potential based molecular dynamics (MD) simulations. The additive pairwise force field (FF) for EMSO and DESO was proposed for the first time, preserving explicit compatibility with their most known homologue, DMSO. The simulations reveal similar structures and thermodynamic properties of DMSO, DESO and EMSO. However, the transport properties are significantly different: DESO and DMSO are less mobile and an order of magnitude more viscous. Furthermore, dipole reorientation in DESO and EMSO occurs ca. 2–4 times slower than in DMSO at room temperature. This observation favors applications of higher sulfoxides as cryoprotectants and provides a microscopic interpretation of the earlier experimental data.

 Please wait while we load your content...
Please wait while we load your content...