Adsorption of PTCDA and C60 on KBr(001): electrostatic interaction versus electronic hybridization†
Abstract
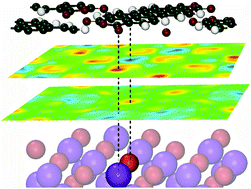
The adsorption of functional molecules on insulator surfaces is of great interest to molecular and organic electronics. Here, we present a systematic investigation of the geometric and electronic properties of perylene-3,4,9,10-tetracarboxylic-3,4,9,10-dianhydride (PTCDA) and C60 on KBr(001) using density functional theory and non-contact atomic force microscopy to reveal the interplay of interactions between aromatic molecules and insulating substrates. Energetic and structural details are discussed, as well as electronic structures, e.g. local electronic density of states, (differential) charge density, and Bader charge analysis, were inspected. Electrostatics was found to be the primary interaction mechanism for systems of PTCDA and C60 adsorbed on KBr, which can be further promoted by electronic hybridizations of non-polar, but polarizable, molecules with substrates, e.g. C60/KBr(001). Electronic hybridization, depending on the polarizability of the π-system, may be suppressed by introducing high electron affinity atoms, e.g. O, into the molecule. Besides, we investigate molecules adsorbed on two-layer KBr(001) covered Cu(001), in which no hybridisation was found between PTCDA and the metal underneath, but a C–Br–Cu hybridized state in C60/KBr(001)/Cu(001). Since the interaction mechanism is dominated by electrostatics, it is concluded that alkali-halides are interesting and important materials for investigation, due to the minor influence on the molecular electronic structure, which may inspire new research fields of electronics.

 Please wait while we load your content...
Please wait while we load your content...