Is it possible to reverse aged acetylcholinesterase inhibited by organophosphorus compounds? Insight from the theoretical study
Abstract
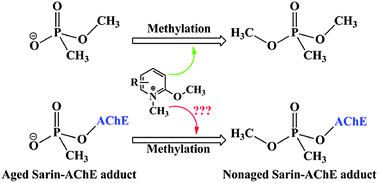
The main treatment for organophosphorus (OP) compound poisoning in clinics is to restore the activity of acetylcholinesterase (AChE) through oxime-induced reactivation of the phosphorylated OP–AChE adduct. It suffers from a competitive and irreversible aging reaction of the phosphorylated OP–AChE adduct, resulting in permanent inactivity of AChE. However, it was recently reported that N-methyl-2-methoxypyridinium species can act as methylating agents to methylate the methyl methane-phosphonate monoanion, in which the reaction mimics the reverse of the aging reaction of the phosphorylated OP–AChE adduct. If the aging reaction could be really reversed, the efficiency for the OP detoxification should be significantly improved, bringing up the possibility to develop an agent to reverse the aging process of the phosphorylated OP–AChE adduct. However, such a reaction with the N-methyl-2-methoxypyridinium species in the enzyme is still not reported so far. It is of great interest to know whether or not this reaction is observable in the enzyme, and more importantly, if it turns out to be not observable in the enzyme, why such a reaction proceeds quickly in aqueous solution but not in the enzyme. In the present study, we performed DFT calculations and quantum mechanical/molecular mechanical (QM/MM) calculations to reveal the fundamental mechanism for the methylation of both the methyl methane-phosphonate monoanion and the aged sarin–AChE adduct by N-methyl-2-methoxypyridinium species, respectively. The obtained results support the SN2 reaction mechanism, not the stepwise mechanism, for the methylation of the methyl methane-phosphonate monoanion by 9 reported N-methyl-2-methoxypyridinium compounds. The calculated free energy barriers are in good agreement with the experimental data. The methylation of the aged sarin–AChE adduct by one N-methyl-2-methoxypyridinium compound (labeled as compound 2) also employs the SN2 reaction mechanism with an extremely high free energy barrier of 30.4 ± 3.5 (or 26.6) kcal mol−1, implying that this reaction in the enzyme hardly occurs. Our results clearly show that compound 2 forms a strong π–π stacking interaction with the aromatic ring of the W86 residue of AChE, making itself unable to approach sarin for the reverse of the aging process. On the basis of the structure and mechanism, several possible strategies have been suggested for designing methylating agents with higher activity against the aged sarin–AChE adduct.

 Please wait while we load your content...
Please wait while we load your content...