Transitional hydrogen bonds in aqueous perchlorate solution†
Abstract
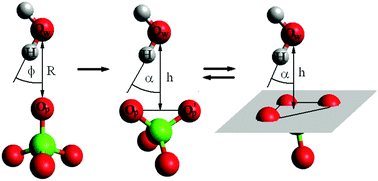
Different mechanisms of H-bond formation between perchlorate anions and water are presented using the molecular dynamics simulations. The detailed methods in searching for multi-centered hydrogen bonds are proposed. The time evolution of H-bond geometric parameters for classical, bifurcated and trifurcated hydrogen bonds in the aqueous perchlorate solution indicates the transitional character of hydrogen bridges as well as the rigid nature of the solvation structure formed by the ion and its first solvation shell. This is supported by the values of free energy binding of water to perchlorate ions determined for particular types of hydrogen bridges.

 Please wait while we load your content...
Please wait while we load your content...