Steric hindrance inhibits excited-state relaxation and lowers the extent of intramolecular charge transfer in two-photon absorbing dyes†
Abstract
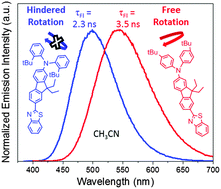
The two-photon absorbing dye AF240 [1, (7-benzothiazol-2-yl-9,9-diethylfluoren-2-yl)diphenylamine] is modified by adding bulky alkyl groups to the diphenylamino moiety. Three new compounds are synthesized which have ethyl groups in both ortho positions of each phenyl ring (2), t-butyl groups in one ortho position of each phenyl ring (3), and t-butyl groups in the para position of each phenyl ring (4). The dyes are examined in several aprotic solvents with varying polarity to observe the effects of the sterically hindering bulky groups on the ground and excited-state photophysical properties. While the ground state shows minimal solvent dependence, there is significant dependence on the fluorescence quantum yield and lifetime, as well as the excited-state energy levels. This effect is caused by the formation of an intramolecular charge-transfer (ICT) state, which is observed in the solvents more polar than n-hexane and supported by TD-DFT calculations. Electronic effects of ortho or para alkyl substitution should be similar, yet drastic differences are observed. A red shift in the fluorescence maximum is observed in 4 relative to 1, yet a blue shift occurs in 2 and 3 because the substituents at the sterically sensitive ortho-positions inhibit excited-state geometric relaxation and result in less ICT character than 1. Coupled with theoretical calculations, the data support a planar ICT (PICT) excited state where the diphenylamino nitrogen in an sp2-like geometry is integral with the plane containing the fluorene and benzothiazole moieties. Ultrafast transient absorption experiments show that ICT occurs rapidly (<150 fs) followed by geometric and solvent relaxation in ∼1–4 ps to form the PICT or solvent-stabilized ICT (SSICT) state. This relaxation is not observed in non-polar n-hexane because the solvent dependent ICT state energy lies higher than the locally-excited (LE) state. Finally, formation of a triplet state (T1) is only efficiently observed in n-hexane for all four dyes.

 Please wait while we load your content...
Please wait while we load your content...