Isolation of pristine MXene from Nb4AlC3 MAX phase: a first-principles study†
Abstract
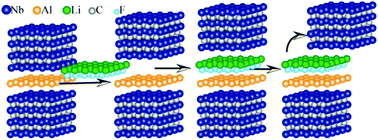
Synthesis of pristine MXene sheets from MAX phase is one of the foremost challenges in getting a complete understanding of the properties of this new technologically important 2D-material. Efforts to exfoliate Nb4AlC3 MAX phase always lead to Nb4C3 MXene sheets, which are functionalized and have several Al atoms attached. Using the first-principles calculations, we perform an intensive study on the chemical transformation of MAX phase into MXene sheets by inserting HF, alkali atoms and LiF in Nb4AlC3 MAX phase. Calculated bond-dissociation energy (BDE) shows that the presence of HF in MAX phase always results in functionalized MXene, as the binding of H with MXene is quite strong while that with F is weak. Insertion of alkali atoms does not facilitate pristine MXene isolation due to the presence of chemical bonds of almost equal strength. In contrast, weak Li–MXene and strong Li–F bonding in Nb4AlC3 with LiF ensured strong anisotropy in BDE, which will result in the dissociation of the Li–MXene bond. Ab initio molecular dynamics calculations capture these features and show that at 500–650 K, the Li–MXene bond indeed breaks leaving a pristine MXene sheet behind. The approach and insights developed here for chemical exfoliation of layered materials bonded by chemical bonds instead of van der Waals can promote their experimental realization.

 Please wait while we load your content...
Please wait while we load your content...