Concentration dependent effects of urea binding to poly(N-isopropylacrylamide) brushes: a combined experimental and numerical study†
Abstract
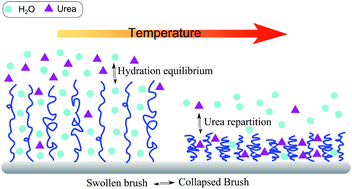
The binding effects of osmolytes on the conformational behavior of grafted polymers are studied in this work. In particular, we focus on the interactions between urea and poly(N-isopropylacrylamide) (PNIPAM) brushes by monitoring the ellipsometric brush thickness for varying urea concentrations over a broad temperature range. The interpretation of the obtained data is supported by atomistic molecular dynamics simulations, which provide detailed insights into the experimentally observed concentration-dependent effects on PNIPAM–urea interaction. In particular, in the low concentration regime (cu ≤ 0.5 mol L−1) a preferential exclusion of urea from PNIPAM chains is observed, while in the high concentration regime (2 ≤ cu ≤ 7 mol L−1) a preferential binding of the osmolyte to the polymer surface is found. In both regimes, the volume phase transition temperature (Ttr) decreases with increasing urea concentration. This phenomenon derives from two different effects depending on urea concentration: (i) for cu ≤ 0.5 mol L−1, the decrease of Ttr is explained by a decrease of the chemical potential of bulk water in the surrounding aqueous phase; (ii) for cu ≥ 2 mol L−1, the lower Ttr is explained by the favorable replacement of water molecules by urea, which can be regarded as a cross-linker between adjacent PNIPAM chains. Significant effects of the concentration-dependent urea binding on the brush conformation are noticed: at cu = 0.5 mol L−1, although urea is loosely embedded between the hydrated polymer chains, it enhances the brush swelling by excluded volume effects. Beyond 0.5 mol L−1, the stronger interaction between PNIPAM and urea reduces the chain hydration, which in combination with cross-linking of monomer units induces the shrinkage of the polymer brush.

 Please wait while we load your content...
Please wait while we load your content...