Ab initio kinetics studies of hydrogen atom abstraction from methyl propanoate†
Abstract
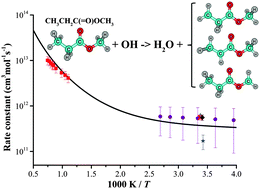
The kinetics of hydrogen abstraction by five radicals (H, CH3, O(3P), OH, and HO2) from a biodiesel surrogate, methyl propanoate (MP), is theoretically investigated. We employ high-level ab initio quantum chemistry methods, coupled-cluster singles and doubles with perturbative triples correction (CCSD(T)) and multi-reference singles and doubles configuration interaction (MRSDCI) with the Davidson-Silver (DS) correction, and obtain chemically accurate reaction energetics. Overall, MRSDCI + DS predicts comparable energetics to CCSD(T) for MP + H/CH3/O/OH. The rate constants are computed using transition state theory (TST-Rice–Ramsperger–Kassel–Marcus theory) in conjunction with the separable-hindered-rotor approximation, variable reaction coordinate TST, and the multi-structure all-structure (MS-AS) approach. A simplified method, semi-multi-structure, is also employed for MP + OH/HO2, and the rate coefficients with this less expensive method are in good agreement with the results obtained with the MS-AS method. The fitted modified Arrhenius expressions are provided over a temperature range of 250 to 2000 K. The predicted rate coefficients for MP + OH agree remarkably well with experimental data over a wide temperature range. Branching ratio analysis of all the studied reactions shows that abstractions of the secondary H atoms within MP are expected to dominate the consumption of fuel at low temperatures, and the contributions of abstractions from the two methyl groups increase with temperature for all abstracting radicals.

 Please wait while we load your content...
Please wait while we load your content...