Ab initio study of the enantio-selective magnetic-field-induced second harmonic generation in chiral molecules
Abstract
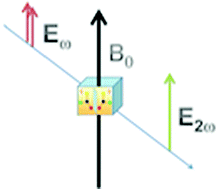
We present a systematic ab initio study of enantio-selective magnetic-field-induced second harmonic generation (MFISHG) on a set of chiral systems ((L)-alanine, (L)-arginine and (L)-cysteine; 3,4-dehydro-(L)-proline; (S)-α-phellandrene; (R,S)- and (S,S)-cystine disulphide; N-(4-nitrophenyl)-(S)-prolinol, N-(4-(2-nitrovinyl)-phenyl)-(S)-prolinol, N-(4-tricyanovinyl-phenyl)-(S)-prolinol, (R)-BINOL, (S)-BINAM and 6-(M)-helicene). The needed electronic frequency dependent cubic response calculations are performed within a density functional theory (DFT) approach. A study of the dependence of the property on the choice of electron correlation, on one-electron basis set extension and on the choice of magnetic gauge origin is carried out on a prototype system (twisted oxygen peroxide). The magnetic gauge dependence analysis is extended also to the molecules of the set. An attempt to analyze the structure–property relationships is also made, based on the results obtained for biphenyl (in a frozen twisted conformation), for prolinol and for some of their derivatives. The strength of the effect is discussed, in order to establish its measurability with a proposed experimental setup.

 Please wait while we load your content...
Please wait while we load your content...