Sodium modified molybdenum sulfide via molten salt electrolysis as an anode material for high performance sodium-ion batteries†
Abstract
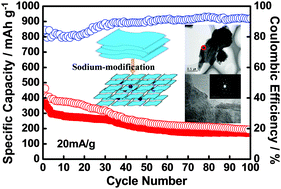
The paper reports a facile and cost effective method for fabricating sodium molybdenum sulfide nanoparticles through using MoS2 sheets as the precursor by sodium-modification. The electrochemical performances of sodium molybdenum sulfide nanoparticles are studied as anode materials for sodium-ion batteries. The galvanostatic charge–discharge measurements have been performed in a voltage range of 0.01–2.6 V vs. Na+/Na under different current densities, using the as-prepared sodium molybdenum sulfide nanoparticles as a working electrode. Typically, the initial discharge and charge capacities of sodium molybdenum sulfide nanoparticles are 475 and 380 mA h g−1, respectively, at a current density of 20 mA g−1. The sodium molybdenum sulfide nanoparticles exhibit high capacity with a reversible discharge capacity of about 190 mA h g−1 after 100 cycles. It should be emphasized that the discharge reaction consists of two steps which correspond to voltage plateaus of 0.93 V and 0.85 V vs. Na+/Na in the first discharge curve of the Na/MoS2 battery, respectively. But there is only one apparent voltage plateau in the Na/Na–Mo–S battery, and it reduces to below 0.5 V vs. Na+/Na, which can enhance the power density. All of the findings demonstrate that sodium molybdenum sulfide nanoparticles have steady cycling performance and environmental and cost friendliness as next generation secondary batteries.

 Please wait while we load your content...
Please wait while we load your content...