Allosteric inhibition of c-Met kinase in sub-microsecond molecular dynamics simulations induced by its inhibitor, tivantinib
Abstract
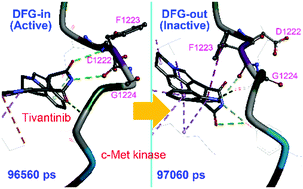
Protein dynamics in the allosteric regulation of enzymes is crucial for understanding the regulation mechanism of enzymes and designing of inhibitors. Kinases have a conserved Asp–Phe–Gly motif (DFG motif) whose conformation determines the activation state of the kinase; however, knowledge on conformational transition of the DFG motif from the active state to the inactive state (“DFG-flip”) is quite limited. Here we report a DFG-flip of c-Met kinase in molecular dynamics (MD) simulations, induced by its allosteric inhibitor tivantinib. Our MD simulations showed that, with the assistance of tivantinib, c-Met may transit from the DFG-in state to the DFG-out state in a sub-microsecond time-scale. A unique binding mode of tivantinib to c-Met was identified to be the key intermediate for the ligand-induced DFG-flip. This study provides a detailed process of inhibitor-induced kinase allostery, as well as important insights into the DFG-flip mechanism and the design of allosteric inhibitors, not only of c-Met, but also of other kinases.

 Please wait while we load your content...
Please wait while we load your content...