Effect of environment on iodine oxidation state and reactivity with aluminum†
Abstract
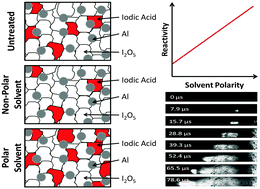
Iodine oxide is a highly reactive solid oxidizer and with its abundant generation of iodine gas during reaction, this oxidizer also shows great potential as a biocidal agent. A problem with using I2O5 in an energetic mixture is its highly variable reactive behavior. This study isolates the variable reactivity associated with I2O5 as a function of its chemical reaction in various environments. Specifically, aluminum fuel and iodine oxide powder are combined using a carrier fluid to aid intermixing. The carrier fluid is shown to significantly affect the oxidation state of iodine oxide, thereby affecting the reactivity of the mixture. Four carrier fluids were investigated ranging in polarity and water miscibility in increasing order from hexane < acetone < isopropanol < water as well as untreated, dry-mixed reactants. Oxidation state and reactivity were examined with experimental techniques including X-ray photoelectric spectroscopy (XPS) and differential scanning calorimetry (DSC). Results are compared with thermal equilibrium simulations. Flame speeds increased with polarity of the fluid used to intermix the powder and ranged from 180 to 1202 m s−1. The I2O5 processed in the polar fluids formed hydrated states of iodine oxide: HIO3 and HI3O8; and, the nonpolar and dry-mixed samples formed: I2O4 and I4O9. During combustion, the hydrated iodine oxides rapidly dehydrated from HIO3 to HI3O8 and from HI3O8 to I2O5. Both steps release 25% of their mass as vapor during combustion. Increased gas generation enhances convective energy transport and accounts for the increase in reactivity seen in the mixtures processed in polar fluids. These results explain the chemical mechanisms underlying the variable reactivity of I2O5 that are a function of the oxide's highly reactive nature with its surrounding environment. These results will significantly impact the selection of carrier fluid in the synthesis approach for iodine containing reactive mixtures.

 Please wait while we load your content...
Please wait while we load your content...