Towards better understanding of C60 organosols†
Abstract
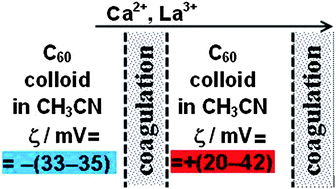
It is of common knowledge that fullerenes form colloids in polar solvents. However, the coagulation via electrolytes and the origin of the negative charge of species are still unexplored. Using a ‘radical scavenger’ and electrospray ionization spectroscopy (ESI), we proved the formation of ion-radical C60˙− and its (probable) transformation into C602− or (C60)22−. The coagulation of C60 organosols by NaClO4 and other perchlorates and nitrates in acetonitrile and its mixture with benzene obeys the Schulze–Hardy rule. At higher Ca(ClO4)2 and La(ClO4)3 concentrations, instead of coagulation, stable re-charged colloidal particles appeared, up to a zeta-potential of +(20–42) mV, as compared with −(33–35) mV of the initial organosols. The influence of both HClO4 and CF3SO3H was similar. This phenomenon is attributed to poor solvation of inorganic cations in cationo- and protophobic acetonitrile, which was proven using [2.2.2] cryptand. Further increasing the concentration of Ca(ClO4)2 led again to coagulation, thus demonstrating a novel type of ‘coagulation zones’.

 Please wait while we load your content...
Please wait while we load your content...