The effect of the metal fragment on the aromaticity and synchronicity of the gold(i)-catalysed divinylcyclopropane–cycloheptadiene rearrangement†
Abstract
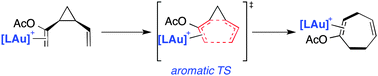
The gold(I)-catalysed divinylcyclopropane–cycloheptadiene rearrangement has been studied computationally within the Density Functional Theory framework. Regardless of the ligand directly attached to the transition metal (L = phosphine, phosphite and N-heterocyclic carbene), the process is found to occur concertedly via endo-boatlike transition structures. The influence of the transition metal fragment on the transformation is analysed and compared to the corresponding uncatalysed process in terms of the computed activation barriers, synchronicity and aromaticity of the associated transition states.
- This article is part of the themed collection: Electron delocalization and aromaticity: 150 years of the Kekulé benzene structure

 Please wait while we load your content...
Please wait while we load your content...