Molecular dynamics study on the nucleation of methane + tetrahydrofuran mixed guest hydrate†
Abstract
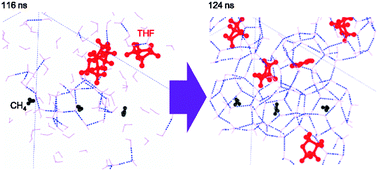
The nucleation of methane (CH4), tetrahydrofuran (THF), and CH4 + THF hydrates are investigated by microsecond MD simulations. These three systems exhibit distinct structural developments in the aqueous phase quantified by the formation of cage structures of hydrogen bonded water molecules. The development of a cluster of cages in the CH4 system is limited by the scarce CH4 molecules in the solution, while in the THF system it is limited by the short lifetime of cages. In the CH4 + THF mixed guest system, a small cluster of caged CH4 molecules can be rapidly stabilized by abundant neighboring cages of THF molecules. Therefore, the induction time of the CH4 + THF mixed guest system is found to be significantly shorter than that of the pure CH4 and pure THF systems. Furthermore, the structure of cages found in the initially formed cage clusters are often different from the typical 5126n (n = 0, 2, 3, 4) cages observed in clathrate hydrate systems. The cluster of cages may grow or transform into structure I or II clathrate hydrate in the later stages.

 Please wait while we load your content...
Please wait while we load your content...