Etching of electrodeposited Cu2O films using ammonia solution for photovoltaic applications†
Abstract
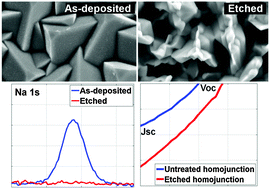
Impurities at the surface of electrodeposited p-Cu2O films have been efficiently removed through the use of concentrated aqueous ammonia solution as a wet etching agent. The performance of the Cu2O homojunction photovoltaic devices incorporating etched p-Cu2O as the bottom layer is higher compared to devices with as-deposited p-Cu2O layers due to an improvement of the homojunction interface quality. Reducing the density of defect states that act as carrier recombination centers led to larger open circuit voltages. The ammonia etchant has been found to preferentially interact with the {100} facets of Cu2O and expose a greater number of {111} facets, resulting in increased interface area for etched homojunction devices, which also improved short circuit current density values.

 Please wait while we load your content...
Please wait while we load your content...