Insights into the reaction mechanism of 3-O-sulfotransferase through QM/MM calculations†
Abstract
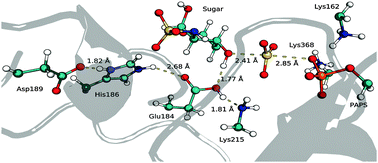
3-O-Sulfotransferase (3-OST) is one of the enzymes involved in heparan sulfate (HS) biosynthesis. HSs are polysaccharides with variable patterns of sulfation and acetylation that serve as entry receptors for herpes simplex virus type 1 (HSV-1). 3-OST is responsible for the transfer of a sulfate group from 3′-phosphoadenosine-5′-phosphosulfate (PAPS) to glucosamine units of HS. In this work, the catalytic mechanism of 3-OST was studied with atomic detail, using computational methods. We investigated the protonation state of key residues using the H++ web-based pKa prediction tool and molecular dynamics (MD) simulations and estimated the most relevant protonation state of the catalytic residues during catalysis. Catalytic histidine (His186) is predominantly protonated, while catalytic aspartate and glutamate (Asp189 and Glu184) are predominantly deprotonated. Subsequently, to study the catalytic mechanism, we applied a QM/MM method at the ONIOM(B3LYP/6-31G(d):ff94) level, starting from three geometries extracted from the 3, 6 and 8 ns point on the MD simulation. The results show that the reaction mechanism of 3-OST occurs by a single elementary step, consisting of an associative SN2 transfer of the sulfate group from PAPS to the HS glucosamine units, with the transfer of a proton from glucosamine to the catalytic Glu184. The activation free energies for this reaction were determined at the ONIOM(M06-2X-D3/6-311++G(2d,2p):ff94//B3LYP/6-31G(d):ff94) level of theory. Despite the free energy differences among the three conformations (10.2, 20.9 and 16.1 kcal mol−1), our results are consistent with the upper limit determined experimentally for the full cycle (20.4 kcal mol−1). The data obtained in this study will be useful for further studies on the inhibition of this enzyme, which is a useful target for drugs that block HSV-1 viral infections.

 Please wait while we load your content...
Please wait while we load your content...