Micro- and nano-tubules built from loosely and tightly rolled up thin sheets†
Abstract
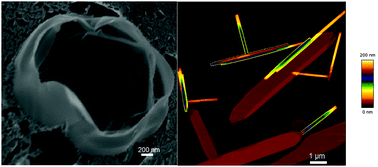
Tubular structures built from amphiphilic molecules are of interest for nano-sensing, drug delivery, and structuring of oils. In this study, we characterized the tubules built in aqueous suspensions of a cholesteryl nucleoside conjugate, cholesterylaminouridine (CholAU) and phosphatidylcholines (PCs). In mixtures with unsaturated PCs having chain lengths comparable to the length of CholAU, two different types of tubular structures were observed; nano- and micro-tubules had average diameters in the ranges 50–300 nm and 2–3 μm, respectively. Using cryo scanning electron microscopy (cryo-SEM) we found that nano- and micro-tubules differed in their morphology: the nano-tubules were densely packed, whereas micro-tubules consisted of loosely rolled undulated lamellas. Atomic force microscopy (AFM) revealed that the nano-tubules were built from 4 to 5 nm thick CholAU-rich bilayers, which were in the crystalline state. Solid-state 2H NMR spectroscopy also confirmed that about 25% of the total CholAU, being about the fraction of CholAU composing the tubules, formed the rigid crystalline phase. We found that CholAU/PC tubules can be functionalized by molecules inserted into lipid bilayers and fluorescently labeled PCs and lipophilic nucleic acids inserted spontaneously into the outer layer of the tubules. The tubular structures could be loaded and cross-linked, e.g. by DNA hybrids, and, therefore, are of interest for further development, e.g. as a depot scaffold for tissue regeneration.


 Please wait while we load your content...
Please wait while we load your content...