Phospholamban spontaneously reconstitutes into giant unilamellar vesicles where it generates a cation selective channel†
Abstract
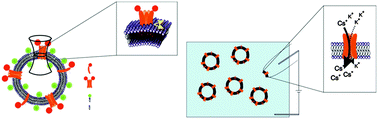
Phospholamban (PLN) is a small integral membrane protein, which modulates the activity of the Sarcoplasmic Reticulum Ca2+-ATPase (SERCA) of cardiac myocytes. PLN, as a monomer, can directly interact and tune SERCA activity, but the physiological function of the pentameric form is not yet fully understood and still debated. In this work, we reconstituted PLN in Giant Unilamellar Vesicles (GUVs), a simple and reliable experimental model system to monitor the activity of proteins in membranes. By Laser Scanning Confocal Microscopy (LSCM) and Fluorescence Correlation Spectroscopy (FCS) we verified a spontaneous reconstitution of PLN into the phospholipid bilayer. In parallel experiments, we measured with the patch clamp technique canonical ion channel fluctuations, which highlight a preference for Cs+ over K+ and do not conduct Ca2+. The results prove that PLN forms, presumably in its pentameric form, a cation selective ion channel.

 Please wait while we load your content...
Please wait while we load your content...