Gas-phase chemistry of ruthenium and rhodium carbonyl complexes
Abstract
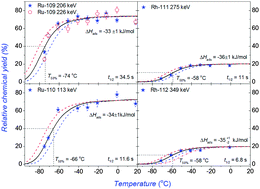
Short-lived ruthenium and rhodium isotopes were produced from a 252Cf spontaneous fission (SF) source. Their volatile carbonyl complexes were formed in gas-phase reactions in situ with the carbon-monoxide containing gas. A gas-jet system was employed to transport the volatile carbonyls from the recoil chamber to the chemical separation apparatus. The gas-phase chemical behaviors of these carbonyl complexes were studied using an online low temperature isothermal chromatography (IC) technique. Long IC columns made up of FEP Teflon were used to obtain the chemical information of the high-volatile Ru and Rh carbonyls. By excluding the influence of precursor effects, short-lived isotopes of 109–110Ru and 111–112Rh were used to represent the chemical behaviours of Ru and Rh carbonyls. Relative chemical yields of about 75% and 20% were measured for Ru(CO)5 and Rh(CO)4, respectively, relative to the yields of KCl aerosols transported in Ar gas. The adsorption enthalpies of ruthenium and rhodium carbonyl complexes on a Teflon surface were determined to be around ΔHads = –33+1−2 kJ mol−1 and −36+2−1 kJ mol−1, respectively, by fitting the breakthrough curves of the corresponding carbonyl complexes with a Monte Carlo simulation program. Different from Mo and Tc carbonyls, a small amount of oxygen gas was found to be not effective for the chemical yields of ruthenium and rhodium carbonyl complexes. The general chemical behaviors of short-lived carbonyl complexes of group VI–IX elements were discussed, which can be used in the future study on the gas-phase chemistry of superheavy elements – Bh, Hs, and Mt carbonyls.

 Please wait while we load your content...
Please wait while we load your content...