Gas-phase structure of 2,2,2-trichloroethyl chloroformate studied by electron diffraction and quantum-chemical calculations†
Abstract
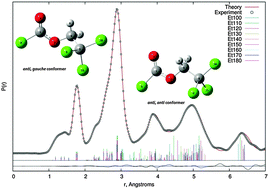
The molecular structure and conformational properties of 2,2,2-trichloroethyl chloroformate, ClC(O)OCH2CCl3 were determined experimentally using gas-phase electron diffraction (GED) and theoretically based on quantum-chemical calculations at the MP2 and DFT levels of theory. Further experimental measurements such as UV-visible, IR and Raman spectroscopy were complemented with the corresponding theoretical studies. All experimental results and calculations confirm the presence of two conformers namely anti–gauche (C1 symmetry) and anti–anti (Cs symmetry). The conformational preference was rationalised by NBO and AIM analyses. Molecular properties such as ionisation potential, electronegativity, chemical potential, chemical hardness and softness were deduced from HOMO–LUMO analyses. The TD-DFT approach was applied to assign the electronic transitions observed in the UV-visible spectrum. A detailed interpretation of the infrared and Raman spectra of the title compound are reported. Using calculated frequencies as a guide, IR and Raman spectra also provide evidence for the presence of both C1 and Cs conformers.

 Please wait while we load your content...
Please wait while we load your content...