R vs. S fluoroproline ring substitution: trans/cis effects on the formation of b2 ions in gas-phase peptide fragmentation†
Abstract
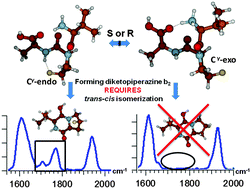
The b2 structures of model systems Xxx-Flp-Ala (Flp = 4R-fluoroproline) and Xxx-flp-Ala (flp = 4S-fluoroproline) (where Xxx is Val or Tyr) were studied by action IRMPD spectroscopy. Proline ring substitutions influence the trans/cis isomerization of the precursor ion, resulting in different b2 fragment ion structures by collision induced dissociation. Vibrational spectra of the b2 ions of Val-Flp and Val-flp exhibit highly intense bands at ~1970 cm−1, revealing that the dominant ion in each case is an oxazolone. The major difference between the spectra of b2 ions for R vs. S fluoroproline is a collection of peaks at 1690 and 1750 cm−1, characteristic of a diketopiperazine structure, which were only present in the 4S-fluoroproline (flp) cases. This suggests only one b2 ion structure (oxazolone) is being formed for Flp-containing peptides, whereas flp-containing peptides produce a mixture of a dominant oxazolone with a lower population of diketopiperazine. In solution, Flp is known to possess a higher trans percentage in the N-terminally adjacent peptide bond, with flp inducing a greater proportion of the cis conformation. The diketopiperazine formation observed here correlates directly with the Ktrans/cis trend previously shown in solution, highlighting that the trans/cis isomerization likelihood for proline residues modified in the 4th position is retained in the gas-phase.

 Please wait while we load your content...
Please wait while we load your content...