Multi-probe relaxation dispersion measurements increase sensitivity to protein dynamics
Abstract
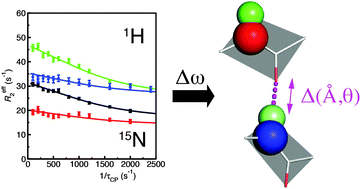
Carr–Purcell–Meiboom–Gill (CPMG) relaxation dispersion measurements are a valuable tool for the characterization of structural transitions on the micro–millisecond timescale. While the measurement of 15N relaxation dispersion is now routine, the measurements with alternative nuclei remain limited. Here we report 15N as well as 1H R2 relaxation dispersion measurements of the N23PP/S148A “dynamic knockout” mutant of dihydrofolate reductase. The 1H dispersion measurements are complementary to 15N data as many additional residues are observed to have dispersive behavior for the 1H nucleus. Simultaneous fitting of the dispersion profiles for the two nuclei increases the accuracy of exchange parameters determined for individual residues and clustered groups of residues. The different sensitivity of the two nuclei to changes in backbone torsional angles, ring currents, and hydrogen bonding effects provides important insights into the nature of the structural changes that take place during the exchange process. We observe clear evidence of direct and indirect hydrogen bond effects for the 15N and 1H chemical shift changes in the active-site, modulation of ring current shielding in the CD-loop and backbone torsional changes in a cluster of residues associated with the C-terminus. This work demonstrates the power of combined 1H and 15N probes for the study of backbone dynamics on the micro-millisecond timescale though the analysis of chemical shift changes.
- This article is part of the themed collection: Exploring the conformational heterogeneity of biomolecules: theory and experiments

 Please wait while we load your content...
Please wait while we load your content...