Anion and cation effects on the size control of Au nanoparticles prepared by sputter deposition in imidazolium-based ionic liquids†
Abstract
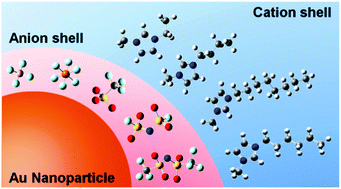
The sputter deposition of metals in an ionic liquid (IL) capture medium is a simple and elegant method for preparing nanoparticles without any chemical reaction. Although there have been some reports on the size determination factors for Au nanoparticles (AuNPs) prepared using this method, the effects with respect to the type of IL used have not been clearly elucidated. This is because there are some complicating factors, some of which have been revealed by our previous systematic studies. In the present study, we prepare AuNPs in nine types of imidazolium-based IL to examine the size determination effects of the type of anion involved, the length of the alkyl chain of the cation, and the preparation temperature for each IL, while keeping other factors constant. For most of the capture media ILs, the sizes of the AuNPs increase with an increase in temperature. The AuNPs prepared in ILs containing different types of anions exhibit distinctly different particle sizes and temperature dependences. Conversely, the alkyl chain is regarded as a secondary stabilizer that works only at higher preparation temperatures. We conclude that the sizes of AuNPs prepared by this method may be determined by the competition between the collision frequency of the ejected Au atoms and the stabilizing capability of the anions that form the first coordination shell around the AuNPs. The AuNP sizes are closely related to the volume of anions.

 Please wait while we load your content...
Please wait while we load your content...