Geometries, interaction energies and complexation free energies of 18-crown-6 with neutral molecules†
Abstract
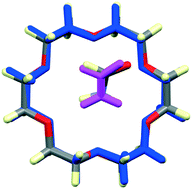
Although 18-crown-6 is renowned for its binding affinity to various metal and ammonium cations, the nature and strength of its binding with neutral guest molecules is relatively unexplored. Here we report a computational study of the host : guest geometries, interaction energies and Gibbs free energies of formation of 18-crown-6 with 49 neutral guest molecules in the gas phase, using the G4(MP2) composite method. Optimized geometries are in excellent agreement with those observed in crystals, with differences readily attributed to guest : guest interactions in the solid state. Host : guest interaction energies range from −13 to −103 kJ mol−1, and the estimated Gibbs free energies of binding at 298 K correlate with the observation (or not) of the complexes in crystals. The electrostatic, dispersion, polarization and repulsion components of the interaction energy have also been estimated using the recently described CE-B3LYP model energies, providing insight into the binding nature between 18C6 and neutral molecules.


 Please wait while we load your content...
Please wait while we load your content...