Iodine sequestration by thiol-modified MIL-53(Al)†
Abstract
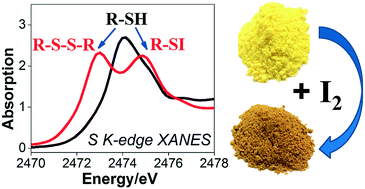
A thiol-modified version of the porous metal organic framework MIL-53 is synthesised in a single step using the functionalised linker precursor 2,5-dithiol-1,4-benzenedicarboxylic acid and aluminium as the framework metal. Careful washing is needed to remove unreacted and dimerised linker from the material after synthesis, but once performed profile fitting of powder X-ray diffraction shows that thiol-modified MIL-53(Al) presents a closed, narrow-pore, structure with unit cell volume ∼1103 Å3 (space group C2/c). The presence of intact thiol groups is confirmed using sulfur K-edge XANES spectroscopy and IR spectroscopy, while nitrogen BET surface area analysis and krypton and xenon adsorption isotherms reveal the porosity of the material. The thiol-modified solid is capable of iodine adsorption from the vapour phase and from solution and an equilibrium uptake of ∼325 mg per g is reached, which is higher than other reported modified forms of MIL-53. Infrared spectroscopy shows the disappearance of the S–H stretch after iodine adsorption, while sulfur K-edge XANES shows a complex spectrum, consistent with the formation of sulfenyl iodide but also oxidation of some sulfur to disulfide having occurred. We therefore propose that formation of covalent S–I bonds allows the sequestration of iodine by the porous solid, but that a proportion of the thiol groups are also in close enough proximity for the formation of disulfide links.


 Please wait while we load your content...
Please wait while we load your content...