Intramolecular H⋯S interactions in metal di-(isopropyl)dithiocarbamate complexes†
Abstract
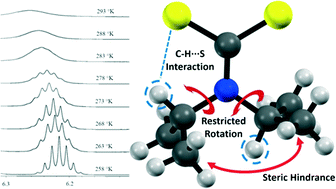
Networks of C–H⋯S interactions have been discovered within the molecular structure of sodium di-(isopropyl)dithiocarbamate pentahydrate with the formula Na(C7H14NS2)·5H2O, revealed by single crystal X-ray diffraction. These interactions have also been investigated by ab initio and Hirshfeld surface analyses which show that the electron density is not symmetrical about the molecule. NMR spectroscopy in solution and solid the state showed temperature dependent restricted rotation of the isopropyl groups, which is attributed to the intramolecular C–H⋯S interactions. The ubiquitous nature of C–H⋯S intramolecular interactions in this class of compound is evident in the structures of other di-(isopropyl)dithiocarbamate complexes deposited in the CSD. In general, the restricted rotation in di-(isopropyl)dithiocarbamate complexes can be directly attributed to intramolecular C–H⋯S interactions, which subsequently influence the geometry in association with steric repulsion factors.


 Please wait while we load your content...
Please wait while we load your content...