Tetra-Fe(iii) and deca-Mn(iii) metallacrowns built from bis-salicylhydrazide ligands: synthesis, structures and magnetic properties†
Abstract
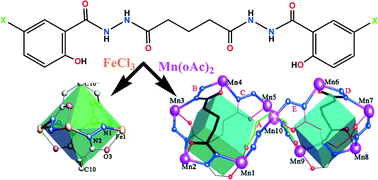
Four new coordination metallacrowns were obtained via self-assembly of Fe(III) and Mn(III) ions with N′1,N′5-bis(5-X-2-hydroxybenzoyl)glutarohydrazide (X = Cl, H6pentaCShz; X = Br, H6pentaBShz) in DMF at room temperature. These metallacrowns consist of four or ten [M–N–N] repeated units with M = Fe(III) for 1 and 2, and M = Mn(III) for 3 and 4. In the systems containing different metal salts, it has been demonstrated that the 12-membered rings in 1 and 2 can be augmented to 30-membered cycles in 3 and 4 due to their metal coordination nature. Quasi-tetrahedra are formed in the tetra-nuclear ferric compounds and two fused distorted tetrahedra are accommodated in the deca-nuclear manganic ones. In other words, the morphologies of the metallacrowns in 1–4 are controlled by varying the metal and flexibility of the glutaryl group in H6pentaXShz. Magnetic studies of all the four compounds indicate antiferromagnetic coupling interactions between adjacent metal centers.

 Please wait while we load your content...
Please wait while we load your content...