Designed synthesis of “L” shaped 17-halo-aryl-ethynyl steroids†
Abstract
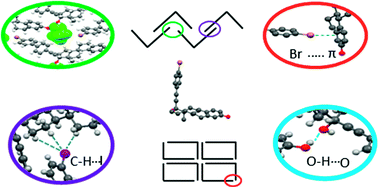
Thirteen steroidal derivatives were synthesized through a Sonogashira cross-coupling reaction which has been found to be an excellent synthetic strategy to introduce halo-aromatic groups into C-17-ethynyl substituted steroidal frameworks. The structural modification was performed on ethynylestradiol, mestranol, norethisterone, ethisterone and 3-ethynyl-3-epi-sarsasapogenin. The solid state study by X-ray diffraction showed that most of them belong to the orthorhombic P212121 space group and the whole family has an “L” conformation, regardless of the nature of the steroid A-ring (3-hydroxy-aromatic or 3-oxo). Due to the presence of several moieties which are susceptible to forming secondary interactions, the crystalline packing is governed by O–H⋯O, C–H⋯O, and C–H⋯π interactions, and only 17α-(4′-iodophenylethynyl)-3-methoxy-estra-1,3,5(10)-trien-17-β-ol (mestranol derivative 3) showed an iodine–iodine interaction (dI⋯I = 4.116 Å). The crystalline packing for ethynylestradiol derivatives 1, 2 and 4 showed the formation of holes with diameters greater than 5.2 Å suggesting their potential application in host guest chemistry or as porous materials.

 Please wait while we load your content...
Please wait while we load your content...