Single-crystal to single-crystal conformational polymorphic transformation in tolbutamide at 313 K. Relation to other polymorphic transformations in tolbutamide and chlorpropamide†
Abstract
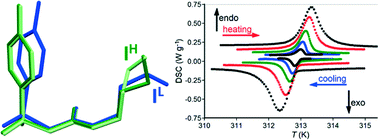
Three polymorphs of tolbutamide (IL, II, and III) were studied using single-crystal X-ray diffraction, from 100 K to room temperature (forms II and III) and to 350 K (form I), and differential scanning calorimetry. The reversible transformation, IL ⇔ IH, was found to be of the single-crystal to single-crystal type and the structure of the high-temperature form (IH) was solved and refined. The structure of IH differs from that of IL only in the conformation of the molecule, with molecular arrangements being practically unchanged (isostructural conformational transformation). The transition takes place at 313 K with no sign of hysteresis. The volume change, ΔV/V, across the reversible transformation IL ⇔ IH was calculated and compared to those for the other conformational transformations. Two types of conformational polymorphic transformations (irreversible reconstructive and reversible isostructural) in tolbutamide and chlorpropamide were compared.

 Please wait while we load your content...
Please wait while we load your content...