Stabilization of polyiodide chains via anion⋯anion interactions: experiment and theory†
Abstract
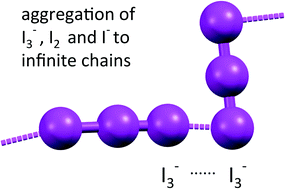
In tyrosinium polyiodide hydrate, cations and anions aggregate in layers. The cation layers are stabilized by classical hydrogen bonds. The anionic part of the structure consists of parallel infinite polyiodide strands; the distance pattern along these chains suggests the presence of smaller subunits I3−, I2 and I−. Comparative calculations for small fragments and longer chains in frozen geometries indicate that this unexpected arrangement is favoured by local stabilizing anion⋯anion interactions and partial charge transfer between the subunits. The topological analyses of the electron density ρ, its negative Laplacian L = −∇2ρ and the electrostatic potential φ functions have been used to study the intrachain I–I and I⋯I interactions. Thorough analysis carried out with L indicates the successive arrangement of generalized charge concentration and charge depletion sites (for either L > 0 or L < 0 regions) along bond paths, and permits to distinguish iodides from iodine atoms even when they are involved in intermediate situations where interatomic distances and net charges are not conclusive.

 Please wait while we load your content...
Please wait while we load your content...