A zeolitic imidazolate framework with conformational variety: conformational polymorphs versus frameworks with static conformational disorder†
Abstract
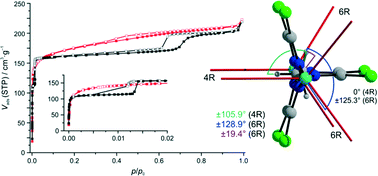
We show via structural considerations and DFT calculations that for a zeolitic imidazolate framework (ZIF) with sodalite (SOD) topology, [Zn(dcim)2]-SOD (dcim = 4,5-dichloroimidazolate), structural models of an infinite number of hypothetical conformational polymorphs with distinct linker orientations can be generated, which can be interconverted most likely only via reconstructive structural transitions. The relative total energies suggest that some of those polymorphs might be synthetically accessible. Efforts in that direction led to the synthesis of new trigonal 1 and previously known cubic 2 with improved crystallinity. According to structural analyses based on powder X-ray diffraction (PXRD) methods supported by NMR spectroscopy, 1 is the most stable of the theoretically predicted SOD-type framework conformers (isostructural to ZIF-7), whereas 2, at variance with a recent proposal, is a SOD-type material with a high degree of orientational disorder of the dcim linker units. The statistics of the linker orientations in 2 is close to that in 1, indicating that the disorder in 2 is not random. Rather, crystals of 2 are likely twins consisting of nanoscopic domains of trigonal 1 that are deformed to a cubic metric, with linker disorder located in the domain interfaces. As structural differences appear to be more related to characteristics of real as opposed to ideal crystal structures, we propose to not consider 1 and 2 as true conformational polymorphs. Systematic investigations of solvent mixtures led to the discovery of intermediate materials of 1 and 2. The PXRD patterns and SEM images indicate that they belong to a complete series of structural intermediates. Differences in the Ar adsorption/desorption behaviours reveal that 1, in contrast to 2, is a flexible ZIF framework.

 Please wait while we load your content...
Please wait while we load your content...