Homochiral crystal generation via sequential dehydration and Viedma ripening†
Abstract
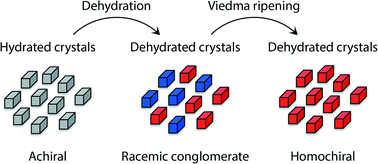
1,2-Bis(N-benzoyl-N-methylamino)benzene (2) forms centrosymmetric hydrate crystals (2·xH2O) and non-centrosymmetric anhydrous crystals. Dehydration of this hydrate (30 min at 140 °C) resulted in the formation of chiral crystals (i.e. a physical racemate of the conglomerate crystals) as verified using solid-state circular dichroism and powder X-ray diffraction. Subsequent attrition-enhanced deracemization, also known as Viedma ripening, was used to obtain homochiral crystals of 2 within 5 h.
- This article is part of the themed collection: 2016 New talent


 Please wait while we load your content...
Please wait while we load your content...