Covalency, hybridization and valence state effects in nano- and micro-sized ZnFe2O4†
Abstract
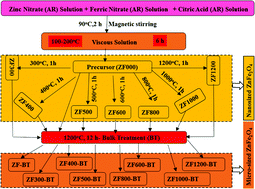
In the present work, Fe valence state, covalency effects, and metal–oxygen hybridization are discussed for ZnFe2O4 using X-ray absorption spectroscopy. A few sets of nano-sized and micro-sized zinc ferrite were synthesized using the nitrate method. Nanoparticles of ZnFe2O4 were synthesized by heating precursor at 300, 400, 500, 800, 1000, and 1200 °C for 1 h. To synthesize micro-sized ZnFe2O4, the obtained nanoparticles were annealed at 1200 °C for 12 h (bulk treatment). X-ray diffraction shows the presence of cubic spinel phase in nano-sized as well as micro-sized ZnFe2O4. Scanning electron microscopy measurements show that particle size ranges are 40–80 nm and 1–2 μm for nano-sized and micro-sized ZnFe2O4, respectively. Fe L-edge spectra of these materials envisage the presence of spectral features corresponding to t2g and eg symmetry states created due to Fe(2p3/2)-Fe(3d) and Fe(2p1/2)-Fe(3d) in octahedral crystal field. This reflects the presence of Fe3+ states in nano-sized and micro-sized ZnFe2O4. eg states dominate in micro-sized ZnFe2O4. O K-edge spectra for these materials can be distinguished by pre-edge and post-edge regions. Pre-edge and post-edge regions are associated with O(2p)–Fe(3d) and O(2p)–Fe(4s,4p) hybridized states. The extent of hybridization estimated from the intensity ratio of O(2p)–Fe(3d) and O(2p)–Fe(4s,4p) hybridized states is higher in nano-sized ZnFe2O4.

 Please wait while we load your content...
Please wait while we load your content...