Synthesis and superior electrochemical properties of shaggy hollow Zn-doped Fe2O3 nanospheres for high-performance lithium-ion batteries†
Abstract
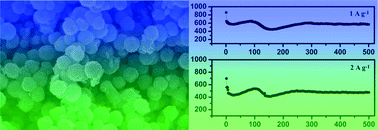
Hollow Zn-doped Fe2O3 nanospheres were fabricated by a facile solvothermal method followed by post-annealing treatment. The as-synthesized hollow nanospheres have a uniform size with a diameter of about 200–500 nm. The growth mechanism of the hollow Zn-doped Fe2O3 nanospheres was proposed based on many contrast experiments. Compared with Fe2O3 without Zn doping, hollow Zn-doped nanospheres showed an improved electrochemical performance in terms of high rate capability and long cycling performance. At current densities of 1 A g−1 and 2 A g−1, Zn-doped Fe2O3 exhibited an initial capacity of 861.5 and 698.4 mA h g−1, respectively, and the capacity was maintained at 580 and 470 mA h g−1 after 500 cycles. These excellent electrochemical properties can be attributed to the unique hollow nanostructure, and Zn doping makes the crystal structure more stable during discharge/charge cycles.

 Please wait while we load your content...
Please wait while we load your content...