Inclusion complexes of Cethyl-2-methylresorcinarene and pyridine N-oxides: breaking the C–I⋯−O–N+ halogen bond by host–guest complexation†
Abstract
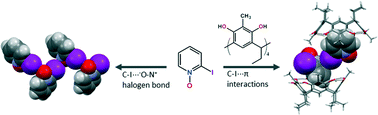
C ethyl-2-Methylresorcinarene forms host–guest complexes with aromatic N-oxides through multiple intra- and intermolecular hydrogen bonds and C–H⋯π interactions. The host shows conformational flexibility to accommodate 3-methylpyridine N-oxide, while retaining a crown conformation for 2-methyl- and 4-methoxypyridine N-oxides highlighting the substituent effect of the guest. N-Methylmorpholine N-oxide, a 6-membered ring aliphatic N-oxide with a methyl at the N-oxide nitrogen, is bound by the equatorial −N–CH3 group located deep in the cavity. 2-Iodopyridine N-oxide is the only guest that manifests intermolecular N–O⋯I–C halogen bond interactions, which are broken down by the host resulting in a 2 : 2 pseudocapsular complex stabilized by additional C–I⋯π interactions between the two 2-iodopyridine N-oxides located in two adjacent hosts. These host–guest complexes were analyzed in the solid state by single crystal X-ray crystallography and in solution by 1H NMR spectroscopy.

 Please wait while we load your content...
Please wait while we load your content...