Structural insights into the coordination chemistry and reactivity of a 3,3′-bis-imine-2,2′-bipyridine ligand†
Abstract
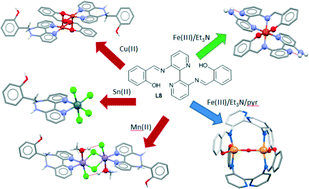
The coordination chemistry of the bis-salicyl-appended Schiff-base ligand L8 with Lewis acidic metal ions affords the mononuclear complex [Sn(L9)Cl4] (1) and two paramagnetic dimers [Cu(L9)(sal)]2(ClO4)2, (2) and [Mn(L9)Cl2(EtOH)]2] (3). The X-ray crystal structures of 1–3 reveal a propensity for L8 to undergo metal catalyzed hydrolysis and cyclization to the diazepine ligand L9. Theoretical calculations on L8 and a model Sn(IV) complex reveal that coordination to a metal ion weakens the imine bonds, rendering them more susceptible to hydrolysis reactions and/or attack by nucleophiles. In contrast, reaction of L8 with FeCl3 in the presence of base affords the partial hydrolysis product [Fe(L10)2]Cl·CH3CN (4). Tuning the reaction conditions via the addition of a second base slows down the hydrolysis of the ligand sufficiently to afford a few crystals of the μ2-oxo diferric complex (μ-O)[Fe(L8)]2·2CH3CN (5) in which intact L8 coordinates to the Fe(III) in a bis-bidentate manner through a deprotonated salicyl oxygen and a bis-imine nitrogen lone pair, with the nitrogen atoms of its 2,2′-bipyridine remaining uncoordinated.


 Please wait while we load your content...
Please wait while we load your content...