Structural, electrochemical and magnetic analyses of a new octanuclear MnIII2MnII6 cluster with linked-defect cubane topology†
Abstract
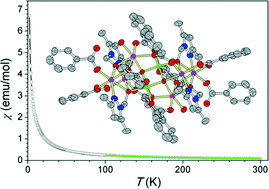
The employment of the 3,5-dimethyl-1-(hydroxymethyl)-pyrazole (Hdmhmp) ligand in a manganese carboxylate cluster afforded the new mixed-valent octanuclear manganese cluster [MnIII2MnII6O2(PhCOO)10(dmhmp)4(H2O)2]·4CH3CN (1). Complex 1 was isolated by the reaction of Mn(ClO4)2·6H2O, Hdmhmp and benzoic acid in a mixed solvent of acetonitrile and methanol. The structure of 1 can be described as a μ4-O2−-linked pair of [Mn4O3] defect cubanes protected by ten PhCO2− and four μ3-dmhmp− ligands. Complex 1 is slightly soluble in acetonitrile, and high-resolution electrospray mass spectrometry (HRESI-MS) indicated that it could keep the [MnIII2MnII6O2] core integrity in solution but with detectable ligand exchange between PhCOO− and dmhmp−. The electrochemical studies show that 1 possesses a characteristic MnII → MnIII oxidation peak at +0.82 V and MnIII → MnII reduction peaks at −0.79 and −1.51 V (versus Fc/Fc+), respectively. A detailed magnetic properties investigation has revealed only a weak intramolecular antiferromagnetic interaction between the MnII and MnIII ions and no characteristic single-molecule magnetic properties.


 Please wait while we load your content...
Please wait while we load your content...